Both respiratory and renal systems are involved in the regulation and maintenance of acid-base balance in blood. In this activity, you will be using respiratory rate, arterial pH, pCO2, and concentration of HCO3– (bicarbonate ion) to diagnose patients with disorders of respiratory alkalosis or acidosis, or metabolic alkalosis or acidosis.
Objectives
- Measure respiratory rates, arterial pH, pCO2, and concentration of HCO3– in four patients with acid-base imbalances.
- Observe how respiratory rate, arterial pH, pCO2,and concentration of HCO3– are used in the diagnosis of conditions of acid-base imbalance.
- Discuss how the respiratory system or renal system (metabolic) will compensate in an attempt to bring the patient back into acid-base balance.
Review
Play the animation to review acid-base balance.
After you have completed this section, click on the Pre-Lab Quiz button at the top menu bar to assess your understanding.
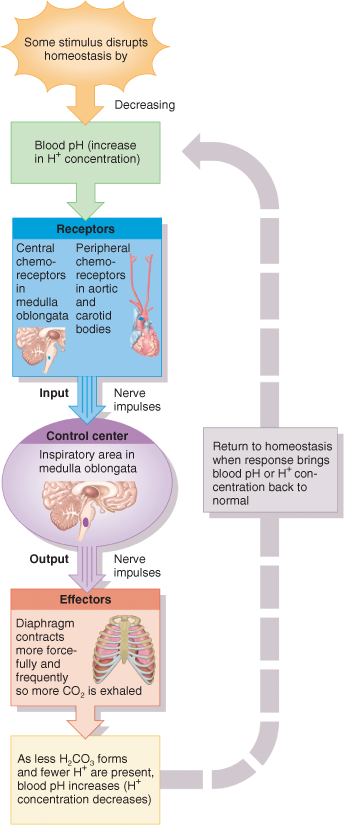
Homeostatic mechanisms maintain blood and body fluid pH at a slightly alkaline pH of 7.35 — 7.45.
There are three major mechanisms at work to maintain the pH of your blood and body fluids within the normal range of 7.35 to 7.45. These mechanisms are:
- Buffer Systems — Temporarily absorb hydrogen ions (H+). This reduces H+ concentration in body solutions and increases pH. Works in seconds.
- Respiratory Systems — Increased exhalation of CO2 or retention of CO2 changes the H+ concentration of body fluids. In the equation, CO2 + H2O → H2CO3 → H+ + HCO3–, when the concentration of one compound decreases, the concentration of the other also changes to maintain the ratio of CO2 to H2CO3 to H+ and HCO3–. When pCO2 in arterial blood increases (retention of CO2), respiration rate increases in an attempt to increase exhalation of CO2. Increased exhalation of CO2 results in decreased concentration of H+ in body fluids and an increased pH. Decreased arterial pCO2 causes respiration rate to decrease in an attempt to increase pCO2. Decreased exhalation of CO2 results in an increased concentration of H+ in body fluids and a decreased pH value. Works in minutes.
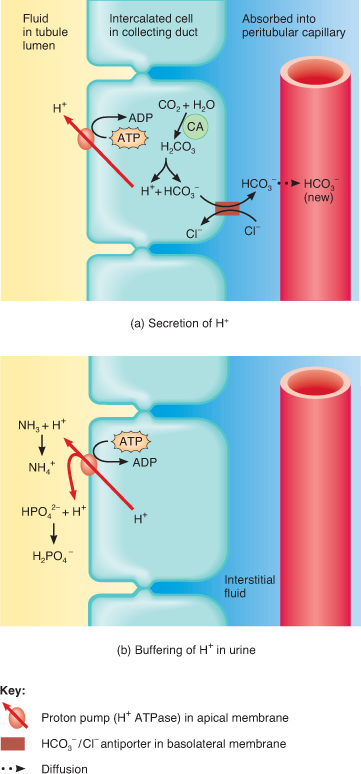
- Kidneys — Secrete excess acids and reabsorbs HCO3–, both of which increase pH. HCO3– binds to H+, and forms H2CO3, removing H+ from solution. Works in hours to days.
Acid-base imbalances occur when arterial blood pH goes outside the normal boundaries. Metabolic processes produce a large quantity of acids (H+), and, without homeostatic mechanisms to lower H+ concentrations, would quickly decrease blood pH. If excess acids are not excreted, the pH of your arterial blood falls below 7.35 and the imbalance is called acidosis. The main physiological effect of acidosis is central nervous system depression. In severe cases, a coma or death can occur.
If an excess amount of acid is excreted or if excess alkaline compounds are ingested, the pH of your arterial blood rises above 7.45 and the imbalance is called alkalosis. The major physiological effect of alkalosis is hypersensitivity and over-stimulation of peripheral nerves and the central nervous system. In severe cases, death occurs.
When acidosis or alkalosis does occur, compensatory mechanisms act to bring arterial blood pH toward normal. If acidosis or alkalosis is due to respiratory disorders, then renal compensation (changes in H+ secretion or HCO3– reabsorption) returns arterial blood pH toward normal. If acidosis or alkalosis is due to metabolic causes, then respiratory compensation (changes in exhalation of CO2) returns arterial blood pH toward normal.
Respiratory Acidosis and Compensation
An abnormally high pCO2 above 45 mmHg and a low pH (below 7.35) in the systemic arterial blood indicates respiratory acidosis. This occurs when the patient hypoventilates or when the patient cannot exhale normally. CO2 accumulates in blood and arterial blood pCO2 increases. This increases the H+ concentration of arterial blood which causes blood pH to decrease. Respiratory acidosis occurs when exhalation of CO2 decreases due to emphysema, chronic bronchitis, airway obstruction, pulmonary edema, injury to respiratory center of medulla (causes hypoventilation), or respiratory muscle disorders.
If the situation is not too challenging, the physiological response is renal compensation that allows the arterial blood pH to return to normal. The kidneys respond by secreting H+ into the kidney tubules and reabsorbing HCO3– back into the arterial blood, allowing the blood pH to increase to normal.
Respiratory Alkalosis
An abnormally low pCO2 below 35 mmHg and a high pH (above 7.45) in the systemic arterial blood indicates respiratory alkalosis. This occurs when the patient starts hyperventilating. Excessive exhalation of CO2, results in decreased pCO2 in arterial blood which lowers concentration of H+. This results in an increase in blood pH. Conditions that cause respiratory alkolosis include: severe anxiety causing hyperventilation, high altitudes with low pO2, pulmonary disease, or stroke.
If the situation is not too challenging, the normal response is renal compensation that allows the arterial pH to decrease to normal. The kidneys respond by reabsorbing H+ into the arterial blood, and secreting HCO3– from the blood and into kidney tubules.
Metabolic Acidosis
An abnormally low level of arterial blood HCO3– (bicarbonate ion) below 22 mEq/liter and a low blood pH (under 7.35) indicates metabolic acidosis. When arterial blood HCO3– is decreased, more H+ remain in solution causing blood pH to decrease. Conditions that cause metabolic acidosis are: severe diarrhea (loss of HCO3–), renal disfunction, ketoacidosis (build up of acidic ketones, also called ketosis), excessive ingestion of alcohol, overdosing on aspirin (or any salicylate), and excessive exercising (lactic acid buildup).
If the situation is not too challenging, the normal response is respiratory compensation that responds by increasing respiration (hyperventilation), allowing more CO2 to be exhaled, the H+ concentration to decrease, and the arterial pH to increase to normal.
Metabolic Alkalosis
An abnormally high level of arterial blood HCO3– (bicarbonate ion) above 26 mEq/liter and a high blood pH (over 7.45) indicates metabolic alkalosis. Conditions that cause metabolic alkalosis are: vomiting (loss of H+), gastric suctioning, constipation (reabsorption of HCO3–), ingesting excess bicarbonate (antacids or alkaline drugs), certain diuretics, and severe dehydration.
If the situation is not too challenging, the normal response is respiratory compensation that responds by decreasing respiration, allowing higher CO2 and H+ concentrations to be retained and the blood pH to decrease to normal.
Measurement of Minute Respiratory Rate pCO2, pH, and HCO3–
Respiratory rate is determined using an acoustic sensor. Arterial blood samples are needed to measure blood gas levels.
Carbon dioxide gas levels (pCO2) are measured with CO2 potentiometry and pH is measured by pH potentiometry. In potentiometry, electric potentials result from differences between the concentration of measured substances in the experimental (arterial blood) sample and a standard solution. The greater the potential difference, the more carbon dioxide present — and the lower the pH — in the blood sample. Arterial blood HCO3– is calculated from pH and pCO2 levels.